Heating Curve Of Water Simulation Answer Key . use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. The sample is initially ice at 1 atm and −23°c; Why do you think the values are so different? compare the heat of vaporization of water to the heat of fusion of water. this interactive simulation from the american association of chemistry teachers allows students to investigate. Energy required = mass × specific heat capacity × temperature change Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. As heat is added, the temperature of the ice increases linearly with time.
from www.chegg.com
this interactive simulation from the american association of chemistry teachers allows students to investigate. Energy required = mass × specific heat capacity × temperature change use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. As heat is added, the temperature of the ice increases linearly with time. The sample is initially ice at 1 atm and −23°c; compare the heat of vaporization of water to the heat of fusion of water. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Why do you think the values are so different?
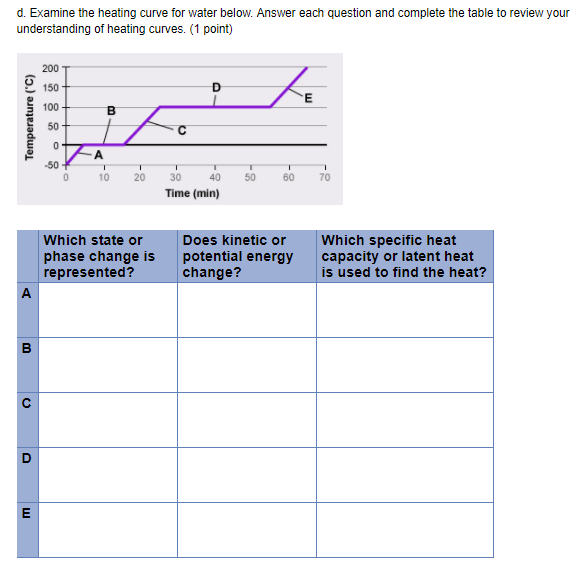
Solved d. Examine the heating curve for water below. Answer
Heating Curve Of Water Simulation Answer Key use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. As heat is added, the temperature of the ice increases linearly with time. use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. compare the heat of vaporization of water to the heat of fusion of water. this interactive simulation from the american association of chemistry teachers allows students to investigate. Why do you think the values are so different? The sample is initially ice at 1 atm and −23°c; Energy required = mass × specific heat capacity × temperature change
From obropolox.blogspot.com
39 heating cooling curve calculations worksheet answers Worksheet Heating Curve Of Water Simulation Answer Key Energy required = mass × specific heat capacity × temperature change Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. this interactive simulation from the american association of chemistry teachers allows students to investigate. The sample is initially ice at 1 atm and −23°c; use the heating. Heating Curve Of Water Simulation Answer Key.
From brainly.com
Examine the heating curve for water below. Answer each question Heating Curve Of Water Simulation Answer Key this interactive simulation from the american association of chemistry teachers allows students to investigate. As heat is added, the temperature of the ice increases linearly with time. use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. Energy required = mass × specific heat capacity × temperature change Why do you. Heating Curve Of Water Simulation Answer Key.
From studylib.net
IB1 Physics Heating Curve of Water Lab Heating Curve Of Water Simulation Answer Key As heat is added, the temperature of the ice increases linearly with time. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. compare the heat of vaporization of water to the heat of fusion of water. The sample is initially ice at 1 atm and −23°c; Why do. Heating Curve Of Water Simulation Answer Key.
From classmedialuisa.z13.web.core.windows.net
Heating Curve Worksheet Answer Key Heating Curve Of Water Simulation Answer Key As heat is added, the temperature of the ice increases linearly with time. use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. this interactive simulation from the american association of chemistry teachers allows students to investigate. The sample is initially ice at 1 atm and −23°c; compare the heat. Heating Curve Of Water Simulation Answer Key.
From dxownccws.blob.core.windows.net
Heating Curve Of Water Answers at Tammy Faber blog Heating Curve Of Water Simulation Answer Key this interactive simulation from the american association of chemistry teachers allows students to investigate. As heat is added, the temperature of the ice increases linearly with time. use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. Why do you think the values are so different? Figure \(\pageindex{3}\) shows a heating. Heating Curve Of Water Simulation Answer Key.
From quizzmagickellen99.s3-website-us-east-1.amazonaws.com
Worksheet Heating Curve Of Water Answers Heating Curve Of Water Simulation Answer Key use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. this interactive simulation from the american association of chemistry teachers allows students to investigate. Why do you think the values are so different? The sample is initially ice at 1 atm and −23°c; As heat is added, the temperature of the. Heating Curve Of Water Simulation Answer Key.
From www.bartleby.com
Answered Examine the heating curve for water?… bartleby Heating Curve Of Water Simulation Answer Key Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. this interactive simulation from the american association of chemistry teachers allows students to investigate. use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. Why do you think the values are. Heating Curve Of Water Simulation Answer Key.
From herbalens.blogspot.com
Heating Curve Worksheet Answer Key Herbalens Heating Curve Of Water Simulation Answer Key use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. As heat is added, the temperature of the ice increases linearly with time. The sample is initially ice at 1 atm and −23°c; compare the heat of vaporization of water to the heat of fusion of water. Figure \(\pageindex{3}\) shows a. Heating Curve Of Water Simulation Answer Key.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Heating Curve Of Water Simulation Answer Key The sample is initially ice at 1 atm and −23°c; Why do you think the values are so different? use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. this interactive. Heating Curve Of Water Simulation Answer Key.
From www.chegg.com
Solved The graph above shows the heating curve of water. Use Heating Curve Of Water Simulation Answer Key The sample is initially ice at 1 atm and −23°c; use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. compare the heat of vaporization of water to the heat of fusion of water. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g. Heating Curve Of Water Simulation Answer Key.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Pdf Heating Curve Of Water Simulation Answer Key this interactive simulation from the american association of chemistry teachers allows students to investigate. Energy required = mass × specific heat capacity × temperature change use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. The sample is initially ice at 1 atm and −23°c; Figure \(\pageindex{3}\) shows a heating curve,. Heating Curve Of Water Simulation Answer Key.
From worksheetlistmo.z21.web.core.windows.net
Heating Curve Of Water Worksheets Answers Heating Curve Of Water Simulation Answer Key Energy required = mass × specific heat capacity × temperature change this interactive simulation from the american association of chemistry teachers allows students to investigate. The sample is initially ice at 1 atm and −23°c; As heat is added, the temperature of the ice increases linearly with time. use the heating curve of water simulation to introduce the. Heating Curve Of Water Simulation Answer Key.
From classzoneatchison.z1.web.core.windows.net
Heating Curve Of Water Worksheets Heating Curve Of Water Simulation Answer Key use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. compare the heat of vaporization of water to the heat of fusion of water. As heat is added, the temperature of the ice increases linearly with time. Energy required = mass × specific heat capacity × temperature change Why do you. Heating Curve Of Water Simulation Answer Key.
From printablelibagnames.z13.web.core.windows.net
Heating Curve Of Water Explained Heating Curve Of Water Simulation Answer Key use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. The sample is initially ice at 1 atm and −23°c; this interactive simulation from the american association of chemistry teachers allows students to investigate. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g. Heating Curve Of Water Simulation Answer Key.
From diyworksheet.com
Worksheet Heating Curve Of Water Answers Heating Curve Of Water Simulation Answer Key Why do you think the values are so different? As heat is added, the temperature of the ice increases linearly with time. this interactive simulation from the american association of chemistry teachers allows students to investigate. use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. The sample is initially ice. Heating Curve Of Water Simulation Answer Key.
From printablelibaccuses.z13.web.core.windows.net
Heating Curve Of Water Worksheets Heating Curve Of Water Simulation Answer Key Energy required = mass × specific heat capacity × temperature change use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. this interactive simulation from the american association of chemistry teachers allows students to investigate. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75. Heating Curve Of Water Simulation Answer Key.
From www.docsity.com
THE HEATING CURVE OF WATER Slides Chemistry Docsity Heating Curve Of Water Simulation Answer Key compare the heat of vaporization of water to the heat of fusion of water. The sample is initially ice at 1 atm and −23°c; Why do you think the values are so different? use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. this interactive simulation from the american association. Heating Curve Of Water Simulation Answer Key.
From lessonzonereinhard.z19.web.core.windows.net
Worksheet Heating Curve Of Water Answers Heating Curve Of Water Simulation Answer Key Energy required = mass × specific heat capacity × temperature change Why do you think the values are so different? As heat is added, the temperature of the ice increases linearly with time. this interactive simulation from the american association of chemistry teachers allows students to investigate. compare the heat of vaporization of water to the heat of. Heating Curve Of Water Simulation Answer Key.